Two Chemical Kinetics Models of a Laser
Introduction
Since the 1950's, many devices have been discovered which produce coherent,
monochromatic, electromagnetic radiation by irradiating a sample of solid,
liquid, or gas that is contained in a chamber having dimensions equal to some
multiple of the radiation's wavelength. Known as MASERs or LASERs,
these devices can be modelled in terms of chemical reactions where photons,
atoms, and molecules occupying different energy states are the reactants and
products. In this paper we use the MLAB mathematical modelling program to
develop chemical kinetics models of systems that amplify electromagnetic
radiation by stimulated emission.
The Three State Model
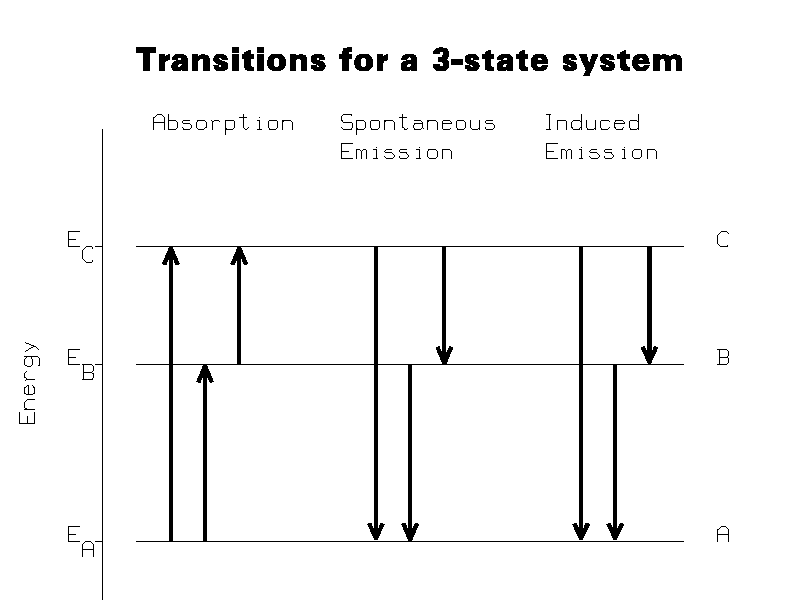
This section refers to a system of atoms which can undergo transitions between
three states. Figure 1 shows the energy levels of the three-state atom, and the
types of transitions that can occur between them. The states, in order of
increasing energy, are designated A, B, and C. The energies of the states are
designated EA, EB, and EC, respectively.

Three types of reactions are seen to occur: absorption reactions, spontaneous emission reactions, and induced (also known as stimulated) emission reactions. The three spontaneous emission reactions are:
| KsCA | ||
| C | ® | A + wCA |
| KsBA | ||
| B | ® | A + wBA |
| KsCB | ||
| C | ® | B + wCB |
The three absorption reactions are:
| KaAC | ||
| A + wCA | ® | C |
| KaAB | ||
| A + wBA | ® | B |
| KaBC | ||
| B + wCB | ® | C |
The three induced emission reactions are:
| KiCA | ||
| C + wCA | ® | A + 2 wCA |
| KiCB | ||
| C + wCB | ® | B + 2 wCB |
| KiBA | ||
| B + wBA | ® | A + 2 wBA |
In these reactions, w represents a photon; its subscript specifies its frequency. For example, wCA is a photon with frequency (EC-EA)/(h/2p) where (h/2p) is Planck's constant. We use K to represent the rate constant for the reaction. The superscripts a, s, and i on K identify whether the rate constant is for an absorption, spontaneous emission, or induced emission reaction, respectively. The subscripts on K identify the reactant and product states involved in the reaction. For example KiBA is the rate constant for the induced emission reaction in which an atom in state B reacts with a photon to produce an atom in state A and two photons.
In a collection of N three-state atoms at equilibrium, the number of atoms in the jth atomic state is given by the Boltzmann distribution,
|
where j is the state label A,B, or C; T is the temperature in degrees Kelvin; N is the total number of atoms in all states; and k is Boltzmann's constant. At room temperature and with the energies we are considering here, the populations of states B and C are negligible when the system is at equilibrium.
In order to generate monochromatic, electromagnetic radiation of frequency wBC by stimulated emission, the collection of three-state atoms must get energy in the form of photons of frequency (EC-EA)/(h/2p). This pumping of the system disrupts the equilibrium distribution of states and creates a population inversion; the number of atoms in state C becomes greater than the number of atoms in state B. When this condition exists, the presence of a photon of frequency wBC catalyzes the the transition from C to B and produces another coherent photon. If the population inversion can be maintained, significant numbers of coherent photons can be produced.
Spontaneous emission reactions follow rate laws that depend only on the number of atoms in the reactant state. Absorption and induced emission reactions follow rate laws that depend on both the number of atoms in the reactant state and the number of reactant photons. From these rules and the nine reactions listed above, we can write six coupled chemical rate equations; one for the population of each of the three states and one for the number of photons at each of the three different frequencies. Using NA(t), NB(t), and NC(t) as the populations of states A, B, and C, respectively, and NAB(t), NBC(t), and NAC(t) as the number of photons having frequency (EB-EA)/(h/2p), frequency (EC-EB)/(h/2p), and frequency (EC-EA)/(h/2p), respectively, the equations are:
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The function pumpAC(t) represents the number of photons per second that are put into the system to pump the ground state A atoms to the state C. The terms kdAB NAB(t) and kdACNAC(t) represent the number of photons per second that are lost due to destructive interference in the chamber and the term kdBCNBC(t) represents the number of wBC photons per second that escape from the chamber.
Using MLAB, we can assign values to the different rate constants, compute the initial Boltzmann distribution of states, define the rate equations, and integrate the rate equations for a continuous wave (CW) laser by executing a script containing the following commands (comments are delimited by "/*" and "*/"):
* assign values for constants */ k = 1.3806E-16 /* Boltzmann's constant in erg/degree Kelvin */ h = 6.6261E-27 /* Planck's constant in erg sec */ EA = 0.1E-11 /* energy of state A in ergs */ EB = 1.05E-11 /* energy of state B in ergs */ EC = 1.06E-11 /* energy of state C in ergs */ N = 1000 /* total number of A,B,C, state atoms */ Np = 10^10 /* total number of photons per second pumped into system */ tmp = 293 /* initial temperature in degrees Kelvin */ kaAB = 1.1e7 /* A->B absorption rate constant in 1/(second*photon) */ kaBC = 1.1e7 /* B->C absorption rate constant in 1/(second*photon) */ kaAC = 1.1e7 /* A->C absorption rate constant in 1/(second*photon) */ ksCA = 1.1e7 /* C->A spontaneous emission rate constant in 1/second */ ksCB = 1.1e7 /* C->B spontaneous emission rate constant in 1/second */ ksBA = 1.1e7 /* B->A spontaneous emission rate constant in 1/second */ kiCA = .1e7 /* C->A induced emission rate constant in 1/(second*atom) */ kiCB = .2e7 /* C->B induced emission rate constant in 1/(second*atom) */ kiBA = .3e7 /* B->A induced emission rate constant in 1/(second*atom) */ kdAB = 1e9 /* B->A photon loss from chamber in 1/second */ kdBC = 1e7 /* C->B photon loss from chamber in 1/second */ kdAC = 1e9 /* C->A photon loss from chamber in 1/second */ /* initial A,B, and C populations from Boltzmann distribution */ fct g(e) = exp(-e/(k*tmp)) bf = g(EA)+g(EB)+g(EC) init NA(0) = N*g(EA)/bf /* atoms */ init NB(0) = N*g(EB)/bf /* atoms */ init NC(0) = N*g(EC)/bf /* atoms */ /* initial BA, CB, and CA numbers of photons */ init NAB(0) = 0 /* photons */ init NBC(0) = 0 /* photons */ init NAC(0) = 0 /* photons */ /* photon rate due to pumping A->C transition */ fct pumpAC(t) = Np /* photons/second */ /* rate equations */ fct NA't(t) = -kaAC*NAC(t)*NA(t)-kaAB*NAB(t)*NA(t)+ksCA*NC(t) +ksBA*NB(t)+kiCA*NAC(t)*NC(t)+kiBA*NAB(t)*NB(t) fct NB't(t) = -kaBC*NBC(t)*NB(t)+kaAB*NAB(t)*NA(t)+ksCB*NC(t) -ksBA*NB(t)-kiBA*NAB(t)*NB(t)+kiCB*NBC(t)*NC(t) fct NC't(t) = -NA't(t)-NB't(t) fct NAB't(t) = -kaAB*NAB(t)*NA(t)+ksBA*NB(t)+kiBA*NAB(t)*NB(t)-kdAB*NAB(t) fct NAC't(t) = pumpAC(t)-kaAC*NAC(t)*NA(t)+ksCA*NC(t)+kiCA*NAC(t)*NC(t) -kdAC*NAC(t) fct NBC't(t) = -kaBC*NBC(t)*NB(t)+ksCB*NC(t)+kiCB*NBC(t)*NC(t)-kdBC*NBC(t) /* integrate the kinetics equations for 1 microsecond */ p = integrate(NA,NB,NC,NAC,NBC,NAB,0:.000001!250) /* columns of p are t NA NA' NB NB' NC NC' NAC NAC' NBC NBC' NAB NAB' */
Here the number of atoms, number of photons, and rate constants have been conveniently chosen so that population inversions are achieved in the allotted time scale. The time dependent population levels of each state and the number of photons at each frequency are graphed by the following commands:
/* draw the time dependence of the number of A's, B's, and C's */ draw p col 1:2 draw p col (1,4) color green draw p col (1,6) color red no framebox top title "population vs. time for CW laser" title t1 = "N'1DA'1U(t)" at (.8,.275) ffract size .01 title t2 = "N'1DB'1U(t)" at (.8,.19) ffract color green size .01 title t3 = "N'1DC'1U(t)" at (.8,.7) ffract color red size .01 frame 0 to 1, .5 to 1 w1=w draw p col (1,8) draw p col (1,10) color green draw p col (1,12) color red no framebox top title "photons vs. time for CW laser" title t5 = "N'1DAC'1U(t)" at (.8,.25) ffract size .01 title t6 = "N'1DBC'1U(t)" at (.8,.75) ffract color green size .01 title t7 = "N'1DAB'1U(t)" at (.85,.16) ffract color red size .01 frame 0 to 1, 0 to .5 view
Figure 2 shows the resulting graph.
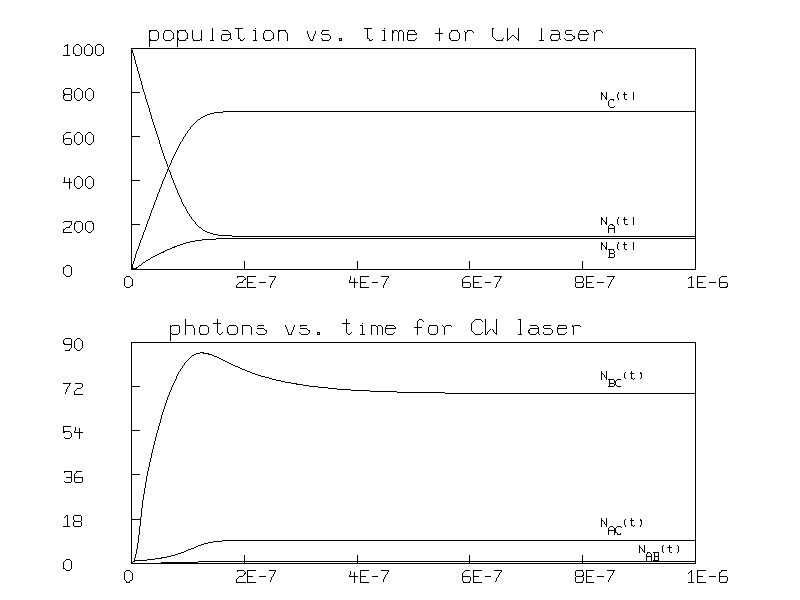
Note that the populations reach steady state after 4 ×10-7 seconds and that the steady state populations are then greater for state C than state B; this is the expected population inversion in a laser.
The MLAB commands above model a laser that operates in a continuous mode; the pump function is constant in time. We can model a pulsed laser by using the previous MLAB commands but with the following definition of the pumping function:
/* define the pulsed input pump function */ fct pumpAC(t) = if (t*10000000-floor(t*10000000) < .5) then 10^10 else 0
This effectively modulates the input so that the pump alternates between 0 and 1 ×1010 photons per second 107 times a second. The resulting populations and numbers of photons are graphed in Figure 3.
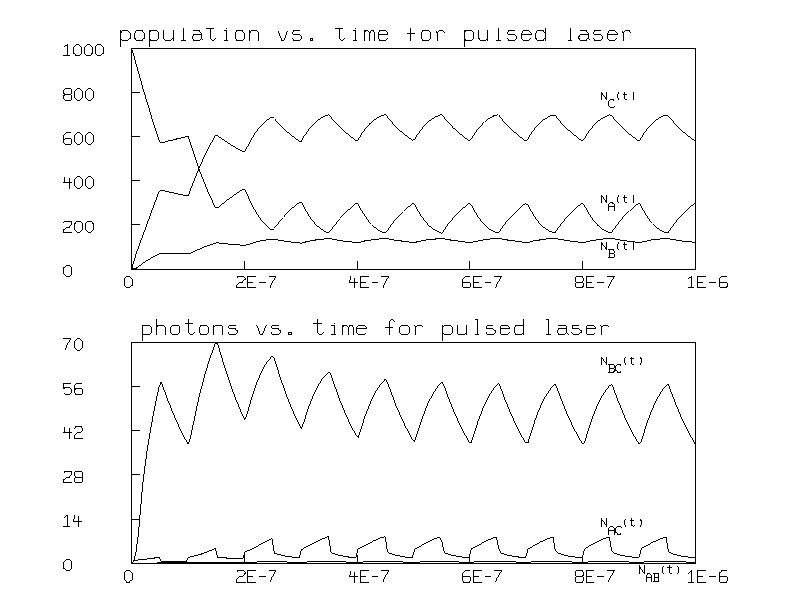
In this figure, the population inversion is seen to be modulated at the frequency of the pump pulses.
An important feature of this three-state model is that one can easily investigate how the population levels change with variations in any of the input constants: N, Np, T, KaAB, KaBC, KaAC, KsCA, KsCB, KsBA, KiCA, KiCB, KiBA, KdAB, KdBC, and KdAC. This makes the model given above an important pedagogical tool.
The Atomic Hydrogen Laser
Another model is based on the lowest 10 levels of basic hydrogen. By the lowest
10 energy levels of basic hydrogen, we mean the 1s, 2s, 2p, 3s, 3p, 3d, 4s, 4p,
4d, and 4f levels one learns about in beginning and intermediate chemistry and
physics courses. Note, this is not a hydrogen MASER, which operates on
the two hyperfine levels of the 1s state. Hydrogen MASERs have been known
experimentally since the late 1950's and are described in Reference 1.
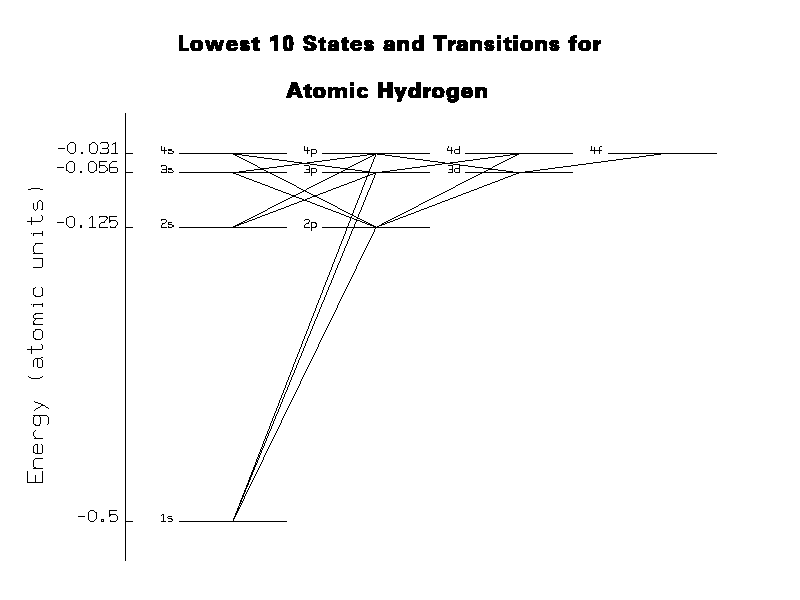
Figure 4 shows the lowest 10 levels of atomic hydrogen and the transitions that are allowed by dipole selection rules. Each line connecting two states represents three reactions: an absorption reaction, a spontaneous emission reaction, and an induced emission reaction.
The spontaneous emission rate constants for this system are calculated exactly from equations (59.11), (63.1), (63.2), and (63.3) of Reference 2. The absorption and induced emission rate constants are calculated from the values of the spontaneous emission rate constants and equations (8.5-1), (8.5-9), and (8.5-10) of Reference 3.
In this computational model, we assume only the ground state is initially populated (Boltzmann factors are not computed); and we continuously pump the 1s ® 4p transition. The decay rate constants, number of photons, and number of atoms, are conveniently selected so that lasing occurs at a frequency of (E4-E3)/(h/2p). Here are the MLAB commands:
/* assign values for constants */
hb = 1.0546E-27 /* Planck's constant divided by 2 pi in erg sec */
c = 2.9979E10 /* speed of light in vacuum (cm/sec)*/
ep = 4.8029E-10 /* proton charge in esu */
em = 9.1096E-28 /* mass of electron in grams */
a0 = 5.2917E-9 /* radius of 1st Bohr orbit in cm */
Z = 1 /* nuclear charge of hydrogen atom */
epau = 4.360E-11 /* ergs per atomic unit of energy */
N = 1e3 /* total number of atoms */
Np = 1e10 /* total number photons per second pumped */
mlt = 1E-15 /* volume proportionality constant relating spontaneous to induced processes */
/* function for evaluating energy of n-th atomic level in ergs */
fct E(n) = -epau*(Z^2)/(2*(n^2))
/* function for evaluating the degeneracy of state with l-orbital angular momentum */
fct g(l) = 2*l+1
/* functions for evaluating spontaneous emission rate constants */
fct f(a,b,c,d) = fact(c-a-b-1)*fact(c-1)*sum(k,max(0,1-c),min(-a,-b),
hgeomd(k,-a,c-a-b-1,-b)*d^k)/(fact(c-b-1)*fact(c-a-1))
fct R(n1,n2,l) = (-1)^(n1-l)*sqrt(fact(n2+l)*fact(n1+l-1))*(4*n1*n2)^(l+1)*\
(n2-n1)^(n2+n1-2*l-2)*(f(-n2+l+1,-n1+l,2*l,-4*n2*n1/(n2-n1)^2)-\
(((n2-n1)/(n2+n1))^2)*f(-n2+l-1,-n1+l,2*l,-4*n2*n1/(n2-n1)^2))/\
((4*fact(2*l-1))*sqrt(fact(n2-l-1)*fact(n1-l))*(n2+n1)^(n2+n1))
fct ks(ni,li,nf,lf) = 4*ep^2*((E(ni)-E(nf))/hb)^3*\
((a0*(if (lf>li) then R(ni,nf,lf) else R(nf,ni,li)))^2)/(3*hb*g(li)*c^3)
/* spontaneous emission rate constants */
ks2p1s = ks(2,1,1,0); ks3p1s = ks(3,1,1,0); ks4p1s = ks(4,1,1,0)
ks3p2s = ks(3,1,2,0); ks4p2s = ks(4,1,2,0); ks4p3s = ks(4,1,3,0)
ks3s2p = ks(3,0,2,1); ks4s2p = ks(4,0,2,1); ks4s3p = ks(4,0,3,1)
ks3d2p = ks(3,2,2,1); ks4d2p = ks(4,2,2,1); ks4d3p = ks(4,2,3,1)
ks4p3d = ks(4,1,3,2); ks4f3d = ks(4,3,3,2)
/* induced emission rate constants */
pch = mlt*PI^2*c^3*hb^2
ki2p1s = pch*ks2p1s/(E(2)-E(1))^3; ki3p1s = pch*ks3p1s/(E(3)-E(1))^3
ki4p1s = pch*ks4p1s/(E(4)-E(1))^3; ki3p2s = pch*ks3p2s/(E(3)-E(2))^3
ki4p2s = pch*ks4p2s/(E(4)-E(2))^3; ki4p3s = pch*ks4p3s/(E(4)-E(3))^3
ki3s2p = pch*ks3s2p/(E(3)-E(2))^3; ki4s2p = pch*ks4s2p/(E(4)-E(2))^3
ki4s3p = pch*ks4s3p/(E(4)-E(3))^3; ki3d2p = pch*ks3d2p/(E(3)-E(2))^3
ki4d2p = pch*ks4d2p/(E(4)-E(2))^3; ki4d3p = pch*ks4d3p/(E(4)-E(3))^3
ki4p3d = pch*ks4p3d/(E(4)-E(3))^3; ki4f3d = pch*ks4f3d/(E(4)-E(3))^3
/* absorption rate constants */
ka1s2p = ki2p1s*g(1)/g(0); ka1s3p = ki3p1s*g(1)/g(0)
ka1s4p = ki4p1s*g(1)/g(0); ka2s3p = ki3p2s*g(1)/g(0)
ka2s4p = ki4p2s*g(1)/g(0); ka3s4p = ki4p3s*g(1)/g(0)
ka2p3s = ki3s2p*g(0)/g(1); ka2p4s = ki4s2p*g(0)/g(1)
ka3p4s = ki4s3p*g(0)/g(1); ka2p3d = ki3d2p*g(2)/g(1)
ka2p4d = ki4d2p*g(2)/g(1); ka3p4d = ki4d3p*g(2)/g(1)
ka3d4p = ki4p3d*g(1)/g(2); ka3d4f = ki4f3d*g(3)/g(2)
/* decay rate constants */
kd12 = 1e12 /* 1<->2 photon loss from resonator in 1/second */
kd13 = 1e12 /* 1<->3 photon loss from resonator in 1/second */
kd14 = 1e12 /* 1<->4 photon loss from resonator in 1/second */
kd23 = 1e12 /* 2<->3 photon loss from resonator in 1/second */
kd24 = 1e12 /* 2<->4 photon loss from resonator in 1/second */
kd34 = 1e4 /* 3<->4 photon loss from resonator in 1/second */
/* initial 1s,2s,2p,3s,3p,3d,4s,4p,4d, and 4f populations */
init N1s(0) = N; init N2s(0) = 0; init N2p(0) = 0
init N3s(0) = 0; init N3p(0) = 0; init N3d(0) = 0
init N4s(0) = 0; init N4p(0) = 0; init N4d(0) = 0
init N4f(0) = 0
/* initial 1<->2, 1<->3, 1<->4, 2<->3, 2<->4, and 3<->4 photons */
init N12(0) = 0; init N13(0) = 0; init N14(0) = 0
init N23(0) = 0; init N24(0) = 0; init N34(0) = 0
/* photon rate due to pumping A->C transition */
fct pump14(t) = Np /* photons/second */\
*(if t<0 then 0 else (1-(gaussd(t,0,1e-9)/gaussd(0,0,1e-9))))
/* define the rate equations */
fct N1s't(t) = -ka1s2p*N1s*N12-ka1s3p*N1s*N13-ka1s4p*N1s*N14
+ki2p1s*N2p*N12+ki3p1s*N3p*N13+ki4p1s*N4p*N14
+ks2p1s*N2p +ks3p1s*N3p +ks4p1s*N4p
fct N2s't(t) = -ka2s3p*N2s*N23-ka2s4p*N2s*N24
+ki3p2s*N3p*N23+ki4p2s*N4p*N24
+ks3p2s*N3p +ks4p2s*N4p
fct N2p't(t) = +ka1s2p*N1s*N12-ka2p3s*N2p*N23-ka2p4s*N2p*N24
-ka2p3d*N2p*N23-ka2p4d*N2p*N24
-ki2p1s*N2p*N12+ki3s2p*N3s*N23+ki4s2p*N4s*N24
+ki3d2p*N3d*N23+ki4d2p*N4d*N24
-ks2p1s*N2p +ks3s2p*N3s
+ks4s2p*N4s
+ks3d2p*N3d +ks4d2p*N4d
fct N3s't(t) = +ka2p3s*N2p*N23-ka3s4p*N3s*N34
-ki3s2p*N3s*N23+ki4p3s*N4p*N34
-ks3s2p*N3s +ks4p3s*N4p
fct N3p't(t) = +ka1s3p*N1s*N13+ka2s3p*N2s*N23-ka3p4s*N3p*N34-ka3p4d*N3p*N34
-ki3p1s*N3p*N13-ki3p2s*N3p*N23+ki4s3p*N4s*N34+ki4d3p*N4d*N34
-ks3p1s*N3p -ks3p2s*N3p +ks4s3p*N4s +ks4d3p*N4d
fct N3d't(t) = +ka2p3d*N2p*N23-ka3d4p*N3d*N34-ka3d4f*N3d*N34
-ki3d2p*N3d*N23+ki4p3d*N4p*N34+ki4f3d*N4f*N34
-ks3d2p*N3d +ks4p3d*N4p +ks4f3d*N4f
fct N4s't(t) = +ka2p4s*N2p*N24+ka3p4s*N3p*N34
-ki4s2p*N4s*N24-ki4s3p*N4s*N34
-ks4s2p*N4s -ks4s3p*N4s
fct N4p't(t) = +ka1s4p*N1s*N14+ka2s4p*N2s*N24+ka3s4p*N3s*N34+ka3d4p*N3d*N34
-ki4p1s*N4p*N14-ki4p2s*N4p*N24-ki4p3s*N4p*N34-ki4p3d*N4p*N34
-ks4p1s*N4p -ks4p2s*N4p -ks4p3s*N4p -ks4p3d*N4p
fct N4d't(t) = +ka2p4d*N2p*N24+ka3p4d*N3p*N34
-ki4d2p*N4d*N24-ki4d3p*N4d*N34
-ks4d2p*N4d -ks4d3p*N4d
fct N4f't(t) = +ka3d4f*N3d*N34-ki4f3d*N4f*N34-ks4f3d*N4f
fct N12't(t) = -ka1s2p*N1s*N12+ki2p1s*N2p*N12+ks2p1s*N2p-kd12*N12
fct N13't(t) = -ka1s3p*N1s*N13+ki3p1s*N3p*N13+ks3p1s*N3p-kd13*N13
fct N14't(t) = pump14(t)-ka1s4p*N1s*N14+ki4p1s*N4p*N14+ks4p1s*N4p-kd14*N14
fct N23't(t) = -ka2s3p*N2s*N23+ki3p2s*N3p*N23+ks3p2s*N3p
-ka2p3s*N2p*N23+ki3s2p*N3s*N23+ks3s2p*N3s
-ka2p3d*N2p*N23+ki3d2p*N3d*N23+ks3d2p*N3d-kd23*N23
fct N24't(t) = -ka2s4p*N2p*N24+ki4p2s*N4p*N24*ks4p2s*N4p
-ka2p4s*N2p*N24+ki4s2p*N4s*N24+ks4s2p*N4s
-ka2p4d*N2p*N24+ki4d2p*N4d*N24+ks4d2p*N4d-kd24*N24
fct N34't(t) = -ka3s4p*N3s*N34+ki4p3s*N4p*N34+ks4p3s*N4p
-ka3p4s*N3p*N34+ki4s3p*N4s*N34+ks4s3p*N4s
-ka3p4d*N3p*N34+ki4d3p*N4d*N34+ks4d3p*N4d
-ka3d4p*N3d*N34+ki4p3d*N4p*N34+ks4p3d*N4p
-ka3d4f*N3d*N34+ki4f3d*N4f*N34+ks4f3d*N4f-kd34*N34
/* integrate the kinetics equations for .001 seconds */
disastersw = -1
p = integrate(N1s,N2s,N2p,N3s,N3p,N3d,N4s,N4p,N4d,N4f,N12,N13,N14,N23,N24, N34,0:.001!250)
/* cols p: T N1s N1s'T N2s N2s'T N2p N2p'T N3s N3s'T
N3p N3p'T N3d N3d'T N4s N4s'T N4p N4p'T
N4d N4d'T N4f N4f'T N12 N12'T N13 N13'T
N14 N14'T N23 N23'T N24 N24'T N34 N34'T */
Here is the graph of the total populations of the N=4 and N=3 levels versus time:
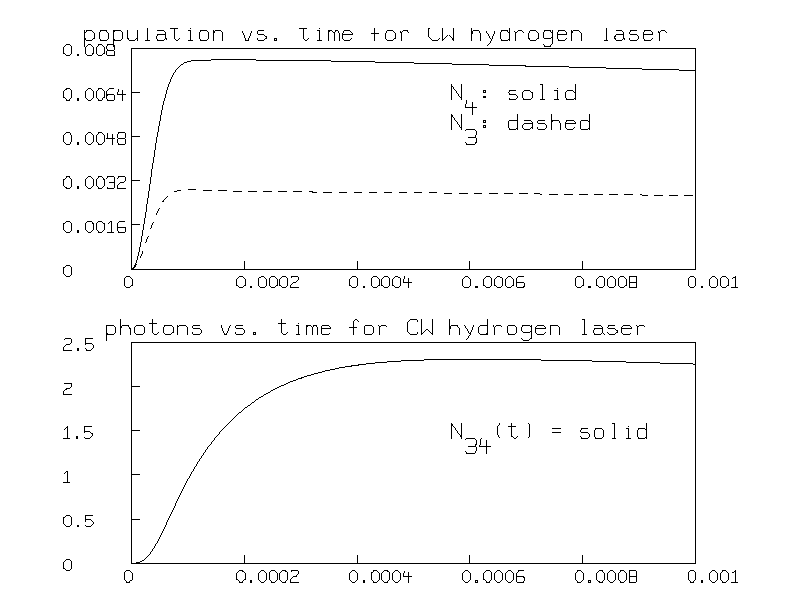
The population inversion is seen to occur after .0005 seconds.
Mathematical models of molecular lasers can be implemented in a manner
similar to that used here to model an atomic laser. Additional terms in
the rate equations may be introduced that account for various radiationless
relaxation processes, such as rotational-to-vibrational relaxation and
electronic-to-vibrational relaxation. A computational limitation does arise in
such mathematical models when there are many coupled rate equations; the
equations become difficult to integrate without numerical errors accruing at
each time step. Such systems of equations are referred to as ill-conditioned
and one can see erroneous exponential growth in one or more of the quantities of
interest. Although MLAB employs several ordinary differential equation-solving
algorithms, including Gear's method for integrating stiff equations, solving
ill-conditioned differential equations over too large an interval will produce
unreliable results.
References
1. Norman F. Ramsey, ``The Atomic Hydrogen Maser'', American Scientist 56
(1968) 420-438.
2. Hans A. Bethe and Edwin E. Salpeter, Quantum Mechanics of One- and Two-Electron Atoms (NY: Plenum Publishing Corp, 1977) 248-269.
3. Amnon Yariv, Quantum Electronics (NY: John Wiley and Sons, 1989) 171-173.